Background:
The treatment outcomes of patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) are significantly poor, especially those who are ineligible for autologous stem cell transplantation (ASCT). R-GemOx (rituximab, gemcitabine and oxaliplatin) regimen is considered to be one of the effective salvage treatments for those pts. Chidamide is an orally active benzamide class of histone deacetylase (HDAC) inhibitor that selectively inhibits activity of HDAC1, 2, 3 and 10. Our preclinical data indicated that chidamide combined with R-GemOx may have potential synergistic anti-tumor effects in DLBCL(unpublished data). An open-label, multicenter, phase 2 study (NCT04022005) was initiated to evaluate the efficacy and safety of chidamide plus R-GemOx(CR-GemOx) regimen in ASCT-ineligible R/R DLBCL. Here we report the preliminary results of this ongoing phase 2 study.
Methods:
Pts aged 18-75 years with ASCT-ineligible R/R DLBCL who failed anthracycline-based chemotherapy were enrolled in this study. The CR-GemOx regimen was administered as follows: chidamide, 20 mg,P.O., twice per week; rituximab 375mg/m2, d1, intravenous drip; gemcitabine 1000mg/m2, d2, intravenous drip; oxaliplatin 100mg/m2, d2,intravenous drip. Repeat cycle every 21 days(up to 6 cycles). Patients achieved complete response (CR) or partial response (PR) were arranged to chidamide maintenance treatment. The primary endpoint was overall response rate (ORR). Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS) and safety. Response was assessed by investigator using CT, MRI or PET-CT every 2 cycles of CR-GemOx treatment or every 8 weeks of chidamide maintenance treatment. The efficacy was evaluated using Lugano 2014 criteria. The safety was assessed according to NCI-CTCAE v5.0.
Results:
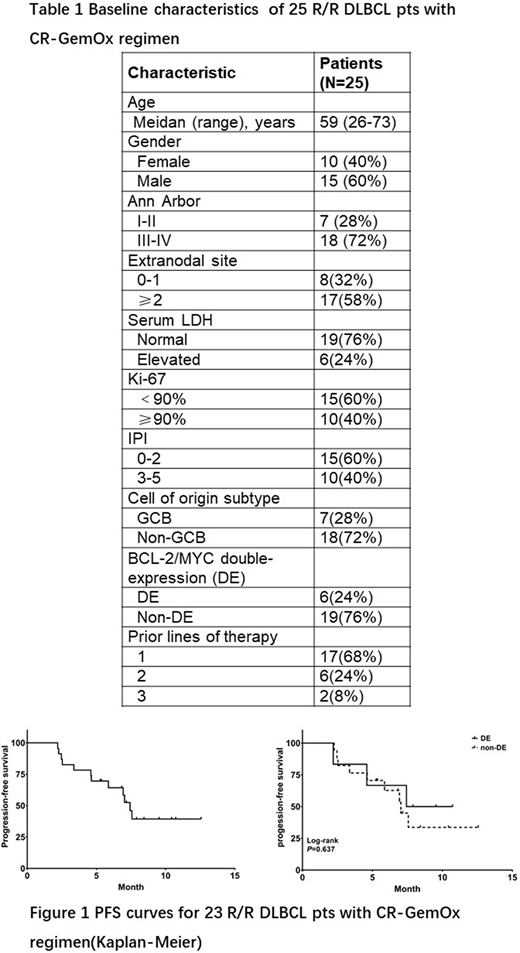
A total of 25 patients were enrolled between August, 2019 and April, 2020, including 13 pts with relapsed diseases and 12 with refractory diseases. Median age was 59 years (range :26-73), 40% were male, median number of prior lines of therapy was 1 (range 1-3). Baseline characteristics are listed in Table 1.For the 23 pts received response assessments, the best ORR was 60.9% and CR rate was 34.8%. Notably, 2 CR and 2 PR were reported in 6 pts with BCL-2/MYC double-expression (DE). With a median follow-up of 8.4 months, the median PFS was 7.4 months (Figure 1). No differences in ORR (66.7% vs 58.8%) and median PFS (7.4 vs 7.0 months) were found between DE and non-DE pts (P > 0.05). Eight pts received chidamide maintenance treatment, and 6 pts still on chidamide maintenance treatment at the cut-off date of June, 2020. Safety was evaluated in 25 pts. The most common (≥20%) treatment-related adverse events (AEs) were thrombocytopenia (60.0%, 15/25), fatigue (60.0%, 15/25), neutropenia (56.0%, 14/25), anemia (56.0%, 14/25), and vomiting (24.0%, 6/25). Grade 3/4 AEs that occurred in more than 2 patients were thrombocytopenia (28.0%, 7/25) and neutropenia (24.0%, 6/25). Dose reductions of chidamide occurred in 8 pts due to AEs (thrombocytopenia, n=5; neutropenia, n=2; diarrhea, n=1).
Conclusions:
The CR-GemOx regimen demonstrates encouraging efficacy in ASCT-ineligible R/R DLBCL pts, including DE pts. Hematologic toxicity is common, particularly thrombocytopenia. Furthermore, lone-term efficacy and safety evaluation in larger cohort is ongoing.
No relevant conflicts of interest to declare.
Chidamide is an orally HDAC inhibitor that has been approved for peripheral T-cell lymphoma. Here we use chidamide plus R-GemOx regimen for ASCT-ineligible relapsed/refratorcy DLBCL patients.
Author notes
Asterisk with author names denotes non-ASH members.